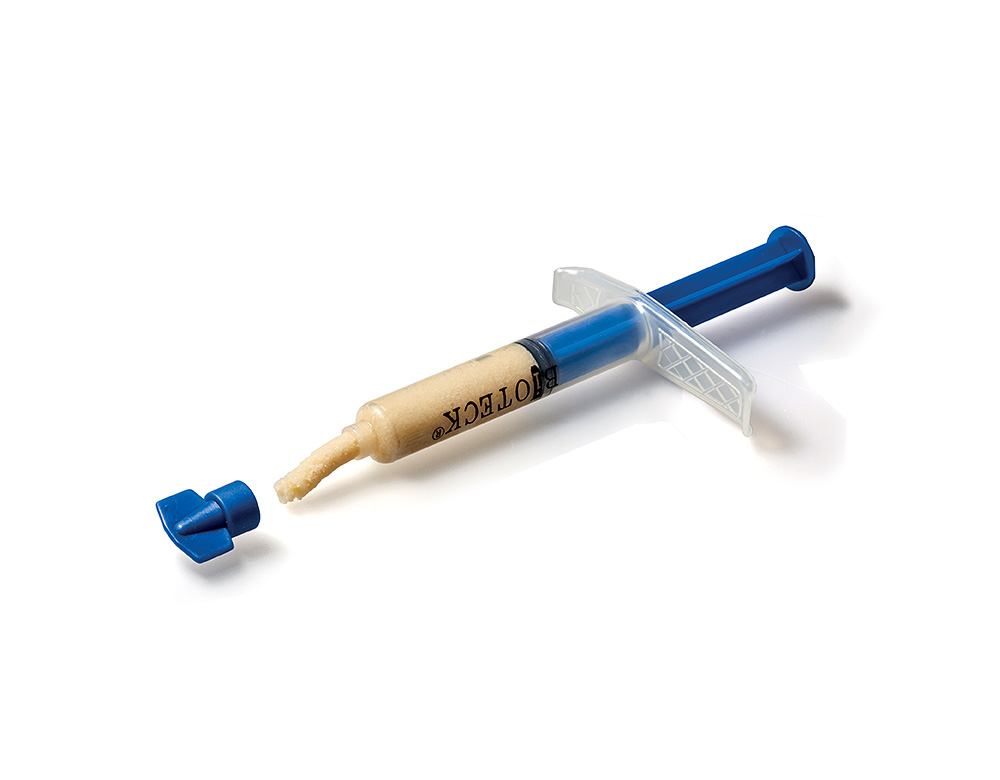
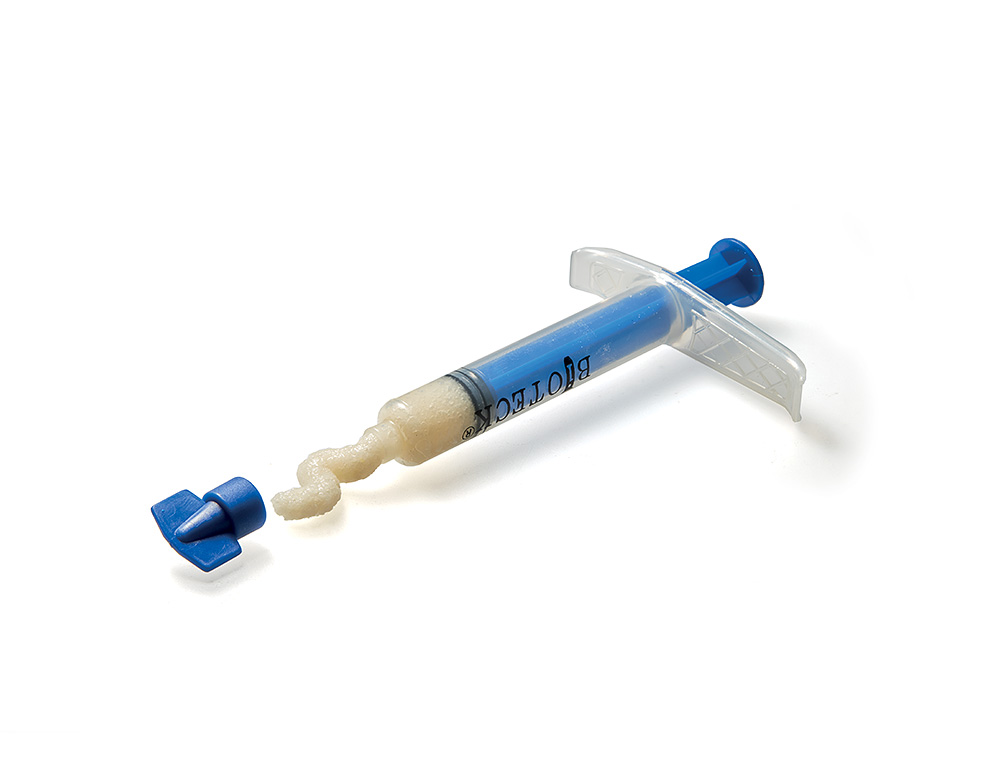
ACTIVABONE

Bone Pastes
منتجات ACTIVABONE لإصلاح العظام هي منتجات شركة BIOTECK في إيطاليا لاستبدال أنسجة العظام التالفة. بسبب التركيبة الجديدة ، يتمتع هذا المنتج بخاصية عالية لتحفيز تكوين العظام. هذا المنتج متوفر بلزوجة ومراحل مختلفة من الهلام ، معجون قابل للحقن ومسيطر ، وقطع ومسحوق العظام.
Activabone® Mouldable Paste
Composition: Demineralized bone matrix (DBM), bone powder, cortical and cancellous granules Ø 0.5-1 mm, type I collagen, Exur® (low molecular weight carrier – LMW, Vitamin C).
Applications: all bone regeneration procedures, in either contained or non contained defects. Pseudarthrosis, consolidation delays, arthrodesis.
Advantages: the osteo-promoting effect of DBM and the specific rheological properties of the carrier are combined with a high osteoconductive effect given by the presence of bone granules. Excellent ease of handling (moldable), does not require hydration (shortening of surgical times), complete remodeling.
ACT-MLD010 – DBM Mouldable Paste – 1 syringe – 2.0 cc
ACT-MLD020 – DBM Mouldable Paste – 1 syringe – 1.0 cc
ACT-MLD050 – DBM Mouldable Paste – 1 syringe – 5.0 cc
ACT-MLD0100 – DBM Mouldable Paste – 1 syringe – 10.0 cc
Activabone® INJECTABLE PASTE
Composition: demineralized bone matrix (DBM), bone powder, type I collagen, Exur® (low molecular weight carrier (LMW), Vitamin C).
Applications: injectable paste osteo-promoter for filling contained bone defects or in percutaneous treatment of pseudarthrosis and consolidation delays.
Advantages: it combines the osteo-promoting effect of DBM with the specific rheological properties of the carrier, high fluidity (easy to extrude), does not require hydration (shortening of surgical times), complete remodeling.
AACT-INJ010 – DBM Injectable Paste – 1 syringe – 2.0 cc
AACT-INJ020 – DBM Injectable Paste – 1 syringe – 1.0 cc
AACT-INJ050 – DBM Injectable Paste – 1 syringe – 5.0 cc
AACT-INJ0100 – DBM Injectable Paste – 1 syringe – 10.0 cc
Activabone® CLX GEL
Composition: Bone powder, cancellous granules Ø 0.5-1 mm, type I collagen, Exur® (low molecular weight carrier – LMW, Vitamin C).
Applications: carrier to improve the texture of bone grafts and cell concentrates.
Advantages: it promotes better graft management and maintenance on site.
ACT-CLX020 – Bone Powder Gel – 1 syringe – 2.0 cc
ACT-CLX050 – Bone Powder Gel – 1 syringe – 5.0 cc
ACT-CLX0100 – Bone Powder Gel – 1 syringe – 10.0 cc
Activabone® Crunch
Composition: Bone powder, cancellous granules Ø 0.5-1 mm, type I collagen, Exur® (low molecular weight carrier – LMW, Vitamin C).
Applications: filling either contained or non contained bone defects, graft in periprosthetic defects.
Advantages: high ease of handling combined with ideal osteoconductive effect. Does not require hydration (shortening of surgical times), complete remodeling.
ACT-CRU010 – Collagen Chips Crunch – 1 syringe – 1.0 cc
ACT-CRU020 – Collagen Chips Crunch – 1 syringe – 2.0 cc
ACT-CRU050 – Collagen Chips Crunch – 1 syringe – 5.0 cc
ACT-CRU0100 – Collagen Chips Crunch – 1 syringe – 10.0 cc